This article is all about sulfated batteries.
Battery sulfation is a big problem in the motive power world.
So, we’re going to give you the tips you need for battery sulfation prevention and restoration.
You’ll learn:
- What sulfation is
- What causes sulfation
- How to prevent sulfation
- How to revive a sulfated battery
- And lots more!
Let’s dive in!
To understand sulfation, we first have to understand how lead-acid batteries work.
Invented in 1860, lead-acid batteries are the most common and widely used type of battery.
Lead-acid batteries are composed of:
- Cells
- Lead plates
- Electrolyte (sulphuric acid and water)
Each cell contains alternating lead and lead oxide plates and separators immersed in the electrolyte.
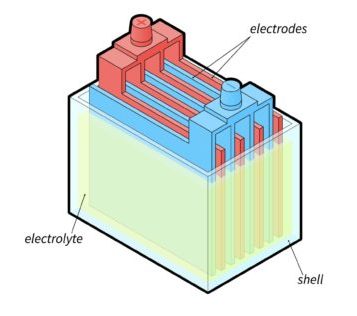
As electrical current flows through the battery, the electrolyte reacts with the lead plates.
When this happens, sulfuric acid in the electrolyte combines with the lead material.
And that turns both plates into lead sulfate.
This is precisely what sulfation is.
Yes, it is.
In fact, sulfation accounts for many early battery failures.
Why?
Because as the battery discharges, the lead sulfate builds up more and more on the plates.
This in turn reduces the surface area of the plates.
As a result, the battery has less active material from which to produce power.
This process can lead to:
Loss of cranking/starting power
Longer charge times
Lower run times between charge times
Shortened battery lifespan
Premature or complete failure
Battery boilovers
Increased heat build-up in the battery
There are two types of battery sulfation:
- Reversible sulfation
- Permanent sulfation
These names imply exactly what each means for the battery.
Reversible sulfation can be reversed.
But you’d need to recognize sulfation early enough for it to be reversed.
If left to accumulate for a long time, it’s highly unlikely you’ll be able to reverse sulfation.
If that happens, the battery will become permanently sulfated.

Also called hard sulfation, permanent sulfation occurs when a battery has been in a low state of charge for a long time, such as weeks or months.
Now, despite the term, permanent sulfation can sometimes be salvaged.
But, it’s highly unlikely.
Sulfation is normal in all batteries.
In fact, it’s a key part of the electrochemical process that generates power.
It’s also only temporary.
That’s because the process is reversed when the battery is recharged.

However, the problem is when the sulfation builds up excessively.
And that can happen if the battery is:
Undercharged
Overcharged
Not in use for a prolonged time (charged or discharged) such as weeks or months
Stored without a full charge
Additionally, you need to be mindful of the temperature that you’re storing or using batteries in.
That’s because temperatures above 75°F will speed up sulfation.
In fact, a battery’s discharge rate doubles, as does sulfation, with every 10°F rise above 75°F.
Now that you know what causes sulfation, it’s easier to understand how to prevent it.
Consider the following suggestions:
Allow the battery to charge fully regularly. And don’t allow it to drop below 12.4 volts
Ensure proper battery storage. Don’t leave batteries discharged and unused for extended periods
Apply a top-up maintenance charge periodically if the battery is in storage
Keep the battery’s temperature below 75°F (24°C)
Perform regular battery maintenance. The less maintenance you do on your battery, the faster lead sulfate crystals will build upon the battery plates
These precautions are key.
Because once excessive sulfation has formed, it may not be possible to reverse it.
If your battery is a victim of sulfation, you’ll notice signs of decreasing battery efficiency.
But, battery efficiency comes from different angles.
So, if your battery is sulfated, you’ll notice some of the following sulfated battery symptoms:
The battery will not charge very well. Or, it simply refuses to charge at all. This is the most common sign of sulfation
The battery will not hold a charge and goes dead long before expected
Your electronic accessories are not getting enough power or amperage. Look for things like dim headlights, slow startup, weak air conditioning, etc.
How to Check if Your Battery is Reversibly or Permanently Sulfated
To know whether you can reverse the sulfation or not, you can check the battery’s voltage discharge curve.
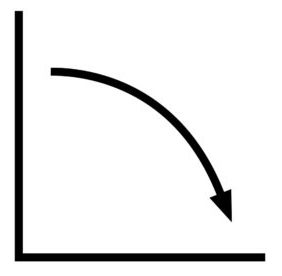
Here’s what to look for:
Make sure the battery is fully charged. Can the battery hold a stable voltage profile on discharge? If it can, it may be reversibly sulfated
Does the voltage drop rapidly with load? Then it’s highly likely that it’s permanently sulfated
What Does a Sulfated Battery Look Like?
Visually, you may not easily notice any difference in battery appearance between a normal and sulfated battery.
But, you can still check a few things.
First, look for a build-up of a white powdery substance on the outside of the battery.

While not a conclusive indication of sulfation, it’s a strong sign.
You can also tell from looking inside of the cells.
Just be careful, because this requires opening up your battery.
And that can expose you to corrosive sulphuric acid and hydrogen fumes.
So it is recommended to do this in a covered, well-ventilated area.
Here are the steps:
-
Carefully remove the caps
-
Look inside each hole to view the battery’s plates, separators, and electrolyte levels
A typical sulfated battery will have grayed and grimy plates.

It may even be hard to distinguish them from each other.
On the other hand, a healthy battery will have clean silver-lead plates.

These should be clearly distinguishable from the black separators.
Battery Sulfation Testing
If you can’t tell the difference, your next best option is to conduct a battery sulfation test.
You can do this by testing the battery’s standing voltage using a multimeter.

Any voltage less than 12.6 volts for an AGM battery indicates that your battery is undercharged.
And that's possibly the result of sulfation.
Remember, you can usually only recover a sulfated battery if it's reversibly sulfated.
So, how do you do it?
There are two methods to bring a reversible sulfated battery back to health:
- Overcharge the battery with high bursts of voltage
- Pulse charge with high-frequency energy
We’ll cover each method in more detail below.
Method 1: Overcharging the Battery With High Bursts of Voltage
The first question you might wonder is: “Can a sulfated battery be charged?”
The answer is yes.
But you’d have to overcharge it if you want to reverse the sulfation.
You can do this by applying high bursts of voltage to an already fully charged battery.
This is done using a battery desulfator, which puts out a regulated current of about 200mA.
Generally, the battery terminal voltage can be allowed to rise to between 2.50 and 2.6 volts per cell (15V and 16V on a 12V monoblock) for around 24 hours.
And that current will help dissolve the lead sulfate crystals.
Also, the overcharge will increase the battery’s temperature to 122–140°F (50–60°C).
And that further helps dissolve the crystals.
Method 2: Pulse Charging With High-Frequency Energy
In this method, you can use a desulfating battery charger to reverse the damage.
Doing so involves using high-frequency electronic pulses to dissolve the crystals.
That’s in contrast to method 1, which uses high voltage.
Here’s how it works...
The pulse charged is followed by a rest period.
Then, sometimes a very short discharging pulse occurs to refresh the discharge.
Using high-frequency pulses can help reverse or delay sulfation and prolong the battery’s life.
But this method is a little controversial.
Why?
Anti-sulfation devices (such as a desulfating battery charger) can apply pulses to battery terminals to prevent and reverse battery sulfation.
But they won’t reverse the damage completely.
So, these devices aren’t always recommended.
Conclusion
There you have it: Our tips for preventing and restoring a sulfated battery.
Now, we’d like to hear from you.
Knowing what you now know, could you fix a sulfated battery?
Let us know in the comments section!